Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kato, Hiroshige*; Sato, Mitsuyoshi*; Owada, Hitoshi*; Mihara, Morihiro;
JNC TN8430 2000-008, 53 Pages, 2000/05
Cementitious materials will be used in TRU waste disposal repository. In such cases, it is considered that the migration of alkaline leachates from cementitious materials, so called high pH plume, will cause dissolution of rock and precipitation of secondary minerals. In addition, the high pH plume will move along the flow of groundwater, so it is predicted that rock formation and components of high pH groundwater vary with time and space. However, time and spatial dependence of the variations of secondary minerals and groundwater components has not been clarified. In order to acquire the data of variations of secondary minerals and groundwater components, we carried out the rock alteration experiments with column method. The crushed granodiorite was filled into 4 meters length column (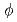 3.7 cm) and artificial cement leachate (pH=13.3; Na=0,1 mol/l, K=0.1 mol/l, Ca=0.002 mol/l) was streamed at flow rates of 0.1 ml/min for 7 months at 80
3.7 cm) and artificial cement leachate (pH=13.3; Na=0,1 mol/l, K=0.1 mol/l, Ca=0.002 mol/l) was streamed at flow rates of 0.1 ml/min for 7 months at 80 C. As the result, secondary minerals confirmed on the rock were calcite and C-S-H at upstream of column and C-S-H at mid-downstream. The pH value of the fluid dominated by Na and K did not be decreased by reaction with the rock. In this study, the data relating to the effect of high pH plume on rock over the long term was acquired.
C. As the result, secondary minerals confirmed on the rock were calcite and C-S-H at upstream of column and C-S-H at mid-downstream. The pH value of the fluid dominated by Na and K did not be decreased by reaction with the rock. In this study, the data relating to the effect of high pH plume on rock over the long term was acquired.
Ashida, Takashi; ; Sato, Haruo; ; Kitamura, Akira; Kawamura, Kazuhiro
JNC TN8400 99-083, 63 Pages, 1999/11
Studies on the chemical and migration behaviour of radionuclides were carried out in the Quantitative Assessment Radionuclide Migration Experimental Facility (QUALITY)for assuring the relaiability and for improving the propriety of data concerning nuclide migration used in the Second Progress Report for the geoloical disposal of high-level radioactive waste. Five studies for solubility, sorption and diffusion concerning nuclide migration were carried out. The overview of each study and the result is as follows: (1)Study on Effect of Carbonate on Np Solubility. Solubilities of Np(IV) were measured as functions of pH and carbonate concentration under reducing conditions. The obtained data could be well described by considering two hydroxo-carbonate complexes, and those stability constants were estimated and compared with the literature data. Consequently, the data obtained in this study were similar to the literature data. (2)Study on Effect of Carbonate on Np Sorption on Bentonite. Distribution coefficients (Kd) of Np(IV) on smectite were measured as a function of carbonate concentration. The obtained Kd values were approximately constant over the carbonate concentration (total carbon concentration 0.04-0.15M). The results of desorption tests by 1M KCl and HCl at the end of sorption experiments showed two different desorption behaviour; Np(IV) was well removed by HCl for the experiments in low carbonate concentration and by KCl for those in high carbonate concentration. (3)Distribution Coefficient Measurements for Cs, Pb and Cm on Rocks. Distribution Coefficients for Cs, Pb and Cm on Japanese major rocks (basalt, mudstone, sandstone, granodiorite and tuff) were measured as a function of ionic strength. The obtained Kd values were either the same orders or higher compared with data used to both fresh and saline groundwater systems in the Second Progress Report. This indicates that the Kd data used in the Second Progress Report are either proper or conservative. ...
Oda, Chie; Ikeda, Takao*; Shibata, Masahiro
JNC TN8400 99-073, 112 Pages, 1999/11
In the safety assessment for geological disposal of high-level radioactive wastes (HLW), distribution coefficients (Kd) and diffusion coefficients of radionuclides are used to estimate the migration of radionuclides in a near-field of repository.  Sn is one of the important nuclides for the safety assessment in Japan and its behavior under reopsitory conditions has not been understood. This report provides the experimental informations for the sorption of Sn on bentonite, tuff and granodiorite, and the diffusion of Sn in a compacted bentonite. The Kd values of Sn on bentonite, tuff and granodiorite were determined by the batch-type sorption experiments as l0
Sn is one of the important nuclides for the safety assessment in Japan and its behavior under reopsitory conditions has not been understood. This report provides the experimental informations for the sorption of Sn on bentonite, tuff and granodiorite, and the diffusion of Sn in a compacted bentonite. The Kd values of Sn on bentonite, tuff and granodiorite were determined by the batch-type sorption experiments as l0
 10
10 [ml/g], 10
[ml/g], 10
 10
10 [ml/g] and 10
[ml/g] and 10
 10
10 [ml/g], respectively. The sequential extraction experiments for adsorbed Sn on bentonite were also performed to investigate its desorption behavior. These experimental results indicated that the mechanisms of sorprion onto bentonite were dominated by the sorption reactions on smectite and pyrite and consisted of reversible and irreversible sorption on solid and stable fixation in solid. On the other hands, the apparent diffusion coefficients (Da) in compacted bentonite were measured by the diffusion experiments as 10
[ml/g], respectively. The sequential extraction experiments for adsorbed Sn on bentonite were also performed to investigate its desorption behavior. These experimental results indicated that the mechanisms of sorprion onto bentonite were dominated by the sorption reactions on smectite and pyrite and consisted of reversible and irreversible sorption on solid and stable fixation in solid. On the other hands, the apparent diffusion coefficients (Da) in compacted bentonite were measured by the diffusion experiments as 10 [m
[m /sec] and l0
/sec] and l0 [m
[m /sec] for dry densities of 0.4[g/cm
/sec] for dry densities of 0.4[g/cm ] and 1.0[g/cm
] and 1.0[g/cm ], respectively. Moreover, the Kd values in compacted bentonite were calculated according to the relationship with the measured Da values, and the solubilities in the porewaters of compacted bentonite were calculated by use of the calculated Kd and the obtained diffusion plofiles. It is found that the derived solubilities almost agreed with the solubiliies of amorphis SnO
], respectively. Moreover, the Kd values in compacted bentonite were calculated according to the relationship with the measured Da values, and the solubilities in the porewaters of compacted bentonite were calculated by use of the calculated Kd and the obtained diffusion plofiles. It is found that the derived solubilities almost agreed with the solubiliies of amorphis SnO reported by Amaya et al. (1997), however, the derived Kd values were lower than that measured from the batch-type sorption experiments.
reported by Amaya et al. (1997), however, the derived Kd values were lower than that measured from the batch-type sorption experiments.
Sato, Haruo
JNC TN8400 99-061, 9 Pages, 1999/10
The porosities and dry densities for rock samples sampled from a fractured zone (fracture type C: composed of intact ganodiorite, altered ganodiorite and fracture fillings) at the Kamaishi mine were obtained by a water saturation (intrusion) method as input parameters for nuclide migration analysis in performance assessment of the geological disposal of high-level radioactive waste. Consequently, the average porosity, 8.6 0.43% was higher than those of fracture fillings, altered garnodiorite and intact ganodiorite composing fracture type B with a single fracture taken from the Kamaishi mine so far. While, the average dry density, 2.43
0.43% was higher than those of fracture fillings, altered garnodiorite and intact ganodiorite composing fracture type B with a single fracture taken from the Kamaishi mine so far. While, the average dry density, 2.43 0.0089 Mg
0.0089 Mg m
m , was lower than those of rocks composing the fracture type B. Based on this, it is predicted that radionuclides are the easiest to migrate in the fracture zone.
, was lower than those of rocks composing the fracture type B. Based on this, it is predicted that radionuclides are the easiest to migrate in the fracture zone.
Sasamoto, Hiroshi; Yui, Mikazu; Arthur, R. C,*
JNC TN8400 99-033, 153 Pages, 1999/07
The results of hydrochemical investigations of groundwaters in the Kurihashi granodiorite at JNC's Kamaishi in-situ tests site indicate that these solutions are: (1)meteoric in origin, (2)chemically reducing (at depths greater than a few hundreds meters), (3)relatively young [residence times in the Kurihashi granodiorite generally less than about 40 years, but groundwaters older than several thousand years BP (before present) are also indicated by preliminary carbon-14 dating of samples obtained from the KH-1 borehole], (4)Ca-HCO type solutions near the surface, changing to Na-HCO
type solutions near the surface, changing to Na-HCO type groundwaters with increasing depth. The evolution of groundwater compositions in the Kurihashi granodiorite is modeled assuming local equilibrium for selected mineral-fluid reactions, taking into account the rainwater origin of these solutions. Results suggest it is possible to interpret approximately the "real" groundwater chemistry (i.e., pH, Eh, total dissolved concentrations of Si, Na, Ca, K, AI, carbonate and sulfate) in the Kurihashi granodiorite if the following assumptions are adopted: (1)CO
type groundwaters with increasing depth. The evolution of groundwater compositions in the Kurihashi granodiorite is modeled assuming local equilibrium for selected mineral-fluid reactions, taking into account the rainwater origin of these solutions. Results suggest it is possible to interpret approximately the "real" groundwater chemistry (i.e., pH, Eh, total dissolved concentrations of Si, Na, Ca, K, AI, carbonate and sulfate) in the Kurihashi granodiorite if the following assumptions are adopted: (1)CO concentration in the gas phase contacting pore solutions in the overlying soil zone = 10
concentration in the gas phase contacting pore solutions in the overlying soil zone = 10 bar, (2)minerals in the rock zone that control the solubility of respective elements in the groundwater include; chalcedony (Si), albite (Na), kaolinite (Al), calcite (Ca and carbonate), microcline (K) and pyrite (Eh and sulfate). Discussions with international experts suggest a systematic approach utilizing reaction-path models of irreversible water-rock interactions in open systems may be needed to more realistically model groundwater evolution at the Kamaishi test site. Detailed information characterizing certain site properties (e.g., fracture mineralogy) may be required to adequately constrain such models, however.
bar, (2)minerals in the rock zone that control the solubility of respective elements in the groundwater include; chalcedony (Si), albite (Na), kaolinite (Al), calcite (Ca and carbonate), microcline (K) and pyrite (Eh and sulfate). Discussions with international experts suggest a systematic approach utilizing reaction-path models of irreversible water-rock interactions in open systems may be needed to more realistically model groundwater evolution at the Kamaishi test site. Detailed information characterizing certain site properties (e.g., fracture mineralogy) may be required to adequately constrain such models, however.
Ueta, Shinzo*
PNC TJ1211 98-002, 46 Pages, 1998/02
None
Ueta, Shinzo*
PNC TJ1211 98-001, 824 Pages, 1998/02
None
*; Shingu, Kazuki*; Takahashi, Eiichiro*; Nakajima, Toshihide*; Yamashita, Mitsugu*; *; *
PNC TJ7187 97-002, 586 Pages, 1997/11
None